
There are a variety of methods that will clean and remove soils from metals. The pretreatment method used is determined by the part to be coated (size, configuration, and material), the type of soil to be removed (dust, wax, and oil) and the performance requirements of the finished product. This process of pretreatment requires three steps. The first two steps are explained the previous pretreatment blog post, below is the final pretreatment step.
CHEMICAL CONVERSION OF THE SURFACE
Phosphating, or conversion coating, is the application of a phosphate coating to the substrate. The most common conversion coatings are iron and zinc phosphate which are applied for the following purposes:
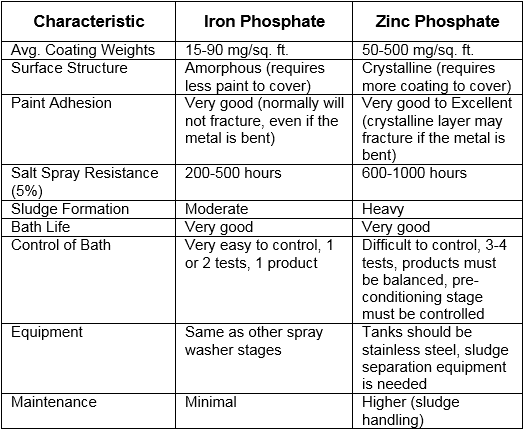
The end product is the most important factor when deciding between iron and zinc phosphate. Iron works well for indoor use in non-corrosive environments and has several economic and environmental advantages. When undercoat protection is needed in a more demanding outdoor, corrosive environment, zinc phosphate is the better option.
Pretreatment is the base for powder coating. The highest quality powder coating will show you excellent results ONLY if the pretreatment is done correctly, maximizing the benefits of powder coating. Oily patches, pin holes, rust spots, reduced resistance to weather, and overall poor powder coating performance will result with improper pretreatment.
The following table compares iron and zinc phosphate processes in more detail.
Have a question about pretreatment? Contact our technical help today for advice from an expert. Or you can always reference our trouble shooting guide online first.